Click here for english version Third update_Final_April 12_JSA & AIPSN
Jan Swasthya Abhiyan (Jsa) And All India People’s Science Network (Aipsn)
Third Update on the Coronavirus Pandemic (Update #3)
April 12th, 2020
This is the third weekly update by JSA-AIPSN. It follows the Background Paper ( http://phmindia.org/wp-content/uploads/2020/03/Background-Paper-COVID19.pdf ) and first JSA-AIPSN Statement (http://phmindia.org/wp-content/uploads/2020/03/Statement-COVID19.pdf ) adopted on March 15th, 2020, the first JSA-AIPSN Update ( https://aipsn.net/2020/04/04/2nd-april-weekly-update-on-covid19-situation/) of March 23rd, 2020 and the second JSA-AIPSN Update (http://phmindia.org/2020/04/03/weekly-update-2-april-the-situation-and-the-peoples-health-movement-response-to-covid19/)of April 2nd, 2020. This update thus covers developments of the last two weeks.
In this document we provide an update on the epidemiology of the corona virus disease (part I), comment on the new public health strategy unveiled this week by Government of India and then discuss the considerations going into the lifting of the lock-down and our position on it (part II). We then present the weekly update of our four working groups looking at: access to essential technologies (part III); health system preparedness (part IV); lockdown restrictions and concerns of rights and ethics (part V) and the crisis in livelihoods and government response to it (part VI).
Part I- Weekly Update on Epidemiology
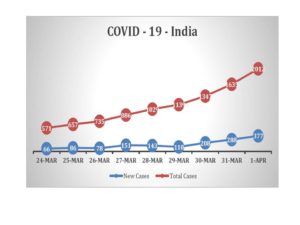
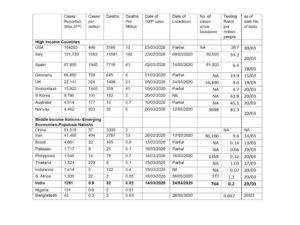
- The current number of cases detected as of 10th April was 6412 (including deaths and cured) which is about 4.9 cases per million population. The number of deaths from COVID-19 is 199, which comes to 0.15 per million population. Reported deaths are 3.1% of reported cases. . The number of deaths is low by international standards, but as pointed out in the last update, this is comparable to figures from many LMIC countries that have such outcomes despite weaker public health systems. (See Annexure 1 pg 15-16 for more details)
- The lower figures in positive cases have largely been due to the lower levels of testing that India undertakes and the much tighter protocol on who can be tested. (All mild , moderate and even mostt severe symptomatic patients were excluded unless they had a histroy of contact or were healthcare providers). However here there is an apparent paradox. As of 9th April, a total of 144,910 tests have been conducted, among the lowest per capita in the world. Internationally, higher test positivity rates are associated with low levels of testing. For instance, South Korea has lowest test positivity rates since it has the widest base of testing. But India’s test positivity rate is around 4.4% , very close to South Korea and well below that of both developed and developing nations. Within India, Kerala has the lowest mortality and test positivity rates. One explanation given for this is that the spread of the virus in India is very limited as compared to any other country, either due to the lock-down or due to perhaps to other hitherto un-determined resistance factors.. We have shown in the past weekly update that the lock down in India was not particularly early and other nations with early lock-down have fared differently. Moreover, the evidence to support resistance factors is very weak.
- A more likely explanation is in the large number of aymptomatic contacts that are tested plus a very low threshold for defining a contact, as well as the systematic exclusion of symptomatic persons with a loack of contact history from testing .. Even substantial numbers of hospitalized persons with severe acute respiratory Infection (SARI) were being excluded from testing. Also, all those who test positive must test negative twice before they are released and this could also add to the number of negative tests. We note that India mainly reports on number of samples tested and has stopped reporting on the number of individuals tested. We cannot be sure that this is the answer, but we suggest that to interpret test positivity rates the disaggregation by indication for testing should be included.
- We are also concerned about the definition being used by Government of India for ‘close contact’. As a result of the current definition, many doctors and nurses who have been transiently in the room are quarantined if a patient who visited their out-patient later tested positive. Or a social worker distributing food packets in a community kitchen is quarantined if one of the beneficiaries with whom they had no direct contact tested positive. In many countries close contact is defined as having been close for at leaast fifteen minutes at a distance of less than six feet and without use of a surgical mask. Singapore has defined it as thirty minutes. If the exposure is shorter than the prescribed limit, but more than two minutes, they can remain on job but would have to wear a mask and have twice daily temperature checks. Other brief incidental contacts are just asked to monitor themselves for symptoms. We are concerned that unnecessary and excessive quarantines will cripple health services and other essential services, while doing little to contain disease, other than the problem of interpretation of aggregated test results. There is an urgent need to rationalize this defnition of close contact, in line with evidence as available, striking an an optimal balance and minimizing quarantine to what is essential. An analysis of the circumstances under which each contact who got the infection from an imported case can be done quite quickly . This was done in Kerala and we know that closed spaces are the highest risk situations. A/C cars, A/C transport and A/C rooms.Further such epidemiological studies are an essential component of any strategy.
- One important unique epidemiological feature of the Indian pandemic is the gender ratio. In almost all nations in the world the ratio of affected men to women is 50%. The range could be 40% to 60%. In India, 76% of those affected are men. The only other nation that has a similar feature is Pakistan with 72% of the affected being men.
Part –II Containment Plan for Large Outbreaks – The new public health strategy
- One major step forward has been the release of the “Containment Plan for Large Outbreaks” by the Ministry of Health and Family Welfare (MOHFW) and in tandem with the new testing strategy announced by ICMR on 9th April.
- The Containment Plan has several welcome features and marks a big step forward. Firstly it goes beyond the “Are we in stage 2 or stage 3?” discussions that had bogged the country down (see Update #2 of April 2nd for our comments on this) and calls for a scenario based approach- and these scenarios varying across the country. It talks of five scenarios instead of stages and lists them as follows:
- Travel related cases reported in India
- Local transmission of COVID-19
- Large outbreaks amenable to containment
- Wide-spread community transmission of COVID-19 disease
- India becomes endemic for COVID-19
- The Plan talks of large outbreaks without specifying whether or not this is community transmission- which, as we discussed before, is quite acceptable and then goes on to detail a strategy of response in this situation. Within this containment area it relaxes testing strategy to all symptomatic influenza like illness (ILI) cases. More importantly it would then limit strict lockdown to such clusters. There is considerable flexibility given to defining the cluster and the features of the lockdown required- allowing for better location-specific response. We note that the plan does not define how wide-spread community transmission situation is defined as compared to large outbreaks- but we can leave it at that. It is note dthat the exit point is being defined as India becoming endemic for the disease, which is realistic, rather than complete elimination.
- JSA-AIPSN calls on the government to build on this plan with the following suggestions and amendments:
- Clarify the strategic objectives and major interventions, not only in scenario III, but also in Scenario IV and V, and how each of these scenarios would be defined and distinguished from each other.
- We demand that a vigorous Covid Disease Surveillance Programme (CDSP) forming a part of the Integrated Disease Surveillance Programme (IDSP) as called for in the first JSA-AIPSN Statement and now elaborated upon) In synergy with a strategy of “Identify-Test-Isolate-Treat-Trace” (ITITT) would be the strategy in scenarios I, II, and V. We clarify that CDSP would be a part of the Integrated Disease Surveillance Programme (IDSP).
- In scenario III and IV also the main strategy would remain CDSP and ITITT, but in addition there would be a small area lock-down in scenario and a state-wide lock-down in scenario IV. It is noted that the measures that constitute “containment” are not limited to any one of the scenarios- but the scale and scope of containment would change. Even those districts and states in sceenario V could revert to one of the other scenarios and rquire containment again.
- The governemnt is also cautioned on using geographic physical distance as measured in kilometers as the main criteria of defining the area under containment. This may have worked in Bhilwara where the main source of infection spread were medical doctors working in a healthcare outpatient department-. Patterns of health-seeking to behaviour are known to correspond to such simplistic circles. But this would not be true if a vendor in a market was the source of spread, or if a gathering of a community or an association was a source of spread, or if it were spreading in an occupational group, or even within a large slum like Dharavi. Thus social mapping would almost always be more important that physical distance as the basis.
- The difference between physical distance mapping and social mapping could become a big problem during implementation. Social mapping to identify disease spread, would require interaction with the communities concerned, and would require social workers to enage in persuasion, negotiation and trust and would further have to depend a lot on ITITT. Lockdowns based on physical distance alone is seen as best enforced by the police department and with the use of force and coercion. Though we concede that there is a considerable overlap between the two, a mechanical and exclusive reliance on physical distance would fail for both epidemiological and societal reasons.
Core Strategy: Identify, Test, Isolate, Treat, Trace (ITITT)
- Recognition on the importance of ITITT as the core strategy is near universal at the policy level. However, there is need to empasise that lock-down is not a substitute for it, but will be needed during and after this and further lock-downs. (Identify= actively seek out symptomatic cases; Test= Test for COVID 19; Isolate: All those who test positive must be placed in isolation, even while waiting to be tested; Treat = All COVID 19 patients who are symptomatic would require treatment based on their severity; Trace= the contacts of all COVID 19 positive patients must be traced based on the patients movement in last 14 days, but especially sine fever had developed and three days before it. These persons would need to be put on quarantining. The main purpose of tracing is on identfying those who need to be on quarantine. Isolation refers to COVID 19 positive patients or symptomatic suspects while waiting for testing. Quarantine is for asymptomatic patients who have had contact and may or may not develop the disease)
- Some states have teams of workers going around and looking for cases of fever and of recent travel outside the town of residence. This seems to be the best way to detect cases early. When fever is detected the team calls in on a line and an ambulance picks up the patient and takes them to a nearby center. If they test positive, they are isolated in earmarked beds and if severe, are taken to dedicated COVID 19 hospitals.. These are the states that are doing well. However, all states need to ensure that workers are protected through adequate PPE and training.
- Some states do all of the above, but their emphasis is more on those with travel history, who they test even if asymptomatic. Unfortunately, those without travel history but with fever are ignored. Many states have even failed to test all those with signs of Severe Acute Respiratory Illness (SARI) if they have no contact or travel history. Often only if an X-Ray or CT scan indicates a problem, and sometimes only half of such patients, are tested even if all are such patients are hospitalized. This is completely unacceptable, but probably happening because these are the protocols currently in place for testing. A combination of lack of testing kits and concerns about detecting high numbers of positive cases which would challenge the states narrative of a great control, combine to cause such deviation.
- A recent ICMR study has shown that in the 5 day interval of March 29th to April 2nd, the number of SARI patients testing for COVID-19 positive increased to 2.6% as compared to 0% in the first two weeks of March and about 1.8% in the latter two weeks of March. Further, of the 104 COVID-19 positive patients about 40% had no history of contact or international travel, which is clearly is community transmission as defined. We also note that the strategy of “large outbreaks amenable to containment” is implcitly an acceptance of communtiy transmission even if it is defined as happenning within a geographically defined hot-spot.
- We now know from case studies of severe patients that they have spent considerable time when they were mild and moderate visiting numerous healthcare providers using public transport, before they were finally diagnosed. The infection spread due to undiagnosed mild and moderate cases is considerable and the clearest reflection of this is the increasing number of healthcare providers who are not in the direct line of COVID-19 duties but are nevertheless coming down (and even one dying) due to COVID-19 infection which they may have got from their general out-patient care. Often it is due to co-morbidities like cancer that patients visit hospitals and pass on the infection to the care providers, such as in the case of the Delhi State Cancer Institute.
- We welcome the steps taken by the government to earmark beds for isolation purposes and hospitals for COVID-19 care in anticipation of a surge of cases. This is most welcome, though we are concerned that where arrangements are of poor quality many patients prefer to escape. We are particularly concerned that train coaches would not be an adequate alternative.
- While steps taken by the Government to step up procurement of ventilators, oxygen supplies, PPE etc, these initiatives have been very late in coming, and also face huge challenges due to transportation disruptions arising from poor implementation of the lockdown. These problems are discussed in the next section.
.
Strengthening Disease Surveillance- CDSP as part of IDSP
- One of the more inexplicable aspects of the government response is the non-use of the integrated disease surveillance programme and within that the flu surveillance programme to track and manage the COVID 19 epidemic. This system is designed to pick up and test for epidemics of respiratory viruses, and could have been easily geared to include COVID19 testing from early February.
- The IDSP system has both event-based reporting and population-based reporting. It provides for suspect-reporting by para-medics, presumptive reporting on clinical crtieria by medical doctors and laboratory based reporting from 168 flu surveillance labs. The 1.5 lakh sub-centers submit S forms (Ssuspected cases) – and they should now be reporting any sudden increase of flu like illness (for which a definition has to be created- like more than 5 fever cases increase in the preceeding week) and any fever releated deaths. But more importantly, all PHCs, CHCs, DH and private cliinics and hospitals should be filling out the P form (Presumptive cases) which should now include a presumptive diagnosis of COVID19 based on clinical crtieria. Such criteria have been proposed by many specialists and are adequate for this purpose. And the laboratories for testing should be expanded from the current 168 to include one or more per district, especially now that rapid tests are available. In the malaria endemic areas, where the malaria surveillance is robust, this may be the preferred framework.
- Any rises of presumptive cases must be followed by a field response team and a testing of a sample of cases if not all cases. In all areas, where COVID 19 is currently at zero levels at least 10% of fever cases must be tested by the rapid test kit with confirmation testing if they test positive. In areas which are reporting increasing COVID cases the testing must try to cover all fever cases. Testing as required for the ITITT strategy would continue.
- Where a lockdown is ordered the testing can include all those with fever and those who are contacts of COVID19, plus those who are asymptomatic and in that area even if they are not close contacts.
- We understand that some states have not invested in their IDSP and many epidemiologist posts lie vacant. No state should consider a lock-down unless they have a minimum CDSP/IDSP in place. Norms for this should be rapidly reiterated and the center step in to close the gaps by transfers, fresh recurits and on the job support as required.
Box: Example of how a Clinical Case definition for presumptive diagnosis could be constructed (based on note by M.S.Seshadri & Jacob John)
Mandatory criterion: Fever of 3 or more days duration without other obvious localizing symptoms (such as dysuria, skin or soft tissue infections)
Epidemiologic setting: Travel within the past 4 weeks to or from any other country or a big crowded city in the country; Visit within the last 4 weeks to a crowded place (bus stand , railway station, movie theatre, airport, place of worship etc)
Major criteria: 1. Dry cough 2. Sudden recent onset Anosmia or loss of taste sensation (anosmia due to nasal block and sinusitis to be excluded 3. Physical findings of crepitations on chest auscultation 4. Chest X Ray showing peripheral patchy infiltrate (not lobar pneumonia or cavitating lesion)
Minor criteria: 1. Diarrhoea 2. Severe headache, body aches (Myalgia) 3. Normal or low normal total WBC count and lymphopenia ( Lymphocytes < 20 % on differential count)
Any persons with a mandatory critiria and at least one major criteria and an epidemiologic setting and/or one more major criteria or one minor crtieria can be taken as presumptive COVID 19. In COVID-19 active areas, this should be enough for isolation even if testing is not available. In other areas, one could self-isolate till testing is done.
On lifting the Lock-Down & Hot-Spots for Containments
- As the National Lock-down period comes to an end, the issue of continuation of lock-down has become the most important topic of discussion. The lockdown is imposing huge economic costs on the majority of the population. This economic shock that majority of the working people have to bear, combining with the virtual suspension of much of essential but routine healthcare could lead to a much greater mortality than the worst case scenario of a corona virus pandemic. However we are concerned that the popular (largely elite and middle class) perception of lockdowns as reflecting strong and determined leadership may push states and the center to re-impose lock-downs all too readily. One view is that lockdowns are being preferred because they can be implemented through a single agency like the police. Many States do not have confidence in their administrative abilities for a multi-dimensional approach that includes rapid scaling up of public health services and manufacture as well as logistics for a variety of essential health commodities, like testing kits and PPEs. A case in point is Odisha which, for a total of 44 positives and one death has decided to lock up 4.6 crore people till April 30th. Only five out of 30 districts test positive but all 30 are locked down. They are the first to announce it and are receiving so much praise for the same. There can be relatively little doubt that deaths due to the withdrawal of other services and the continuing crisis for people’s livelihood would be high. There is also the great possibility that the disease will enter Odisha whenever the lockdown is lifted- say another month from now and peak thereafter. Many other states have also enthusiastically responded to continued lockdowns. Two state task forces/expert committees- Kerala and Karnataka- have recommended a phased withdrawal of lock-downs, but their understanding of phasing is different. The Kerala committee report has important contributions to make, especially with regards to the phased re-introduction of economic activity and further the concept of phased withdrawal / re-introduction of public health measures including travel bans. The challenges are with regards to the criteria of phasing and the data on which this could be measured.
- It is worth noting that both in India, and world over a large number of epidemiologists and those with past experience of epidemic management, and those engaged with health systems strengthening have not quite shown the same enthusiasm for the lock-down. We also note that many nations went for later lock-downs and lifted it when transmission was low, and then returned to lock-downs in areas where transmission aceelerated. This was a part of their strategy, and not a failure of strategy. In this understanding lock-downs are most effective, and the best benefit to loss (pain to gain) ratio, when community transmission is widespread.. However effective a lock-down, whenever that lock-down is lifted, cases will start going up again and one must educate the public and poltiical leadership to understand that this will happen and can be managed. If however the rise crosses a threshold (as measured by a set of indicators that are agreed upon) further area- specific lock-downs could be considered, with adequate preparation and advance notice. But just extending this current lockdown and in general extended lockdowns, will take a huge toll on morbidity and mortality. For example many cancer patients and heart disease patients who needed elective surgery will possibly develop conmplications and start dying if surgey is further postponed. Deaths due to non-COVID causes would rise exponentially with each extension of the lock-down.
- We are agreed that the early lockdown as was imposed now was required for preparing health systems. We also agreed that this lock-down should have been much better prepared for and implemented- and not sprung as a surprise. We however DO NOT agree with the nation-wide extension of the lock-down or even across any State. We think that the current plan for “Large Outbreaks Amenable to Containments” its identification of hotspots is a good way to move forward and state-wide lockdowns should be considered only with wide-spread community transmission of COVID-19 (i.e scenario IV) and which is defined by multiple large outbreaks or clusters or hotspots in all regions within the state.
- Of course lock-down has to be lifted in a phased manner. But the manner of phasing and re-organization of work should have at its core a humane and poor-friendly approach. The arrogance that at times of epidemics we need “stern action” that necessarily will trample on the rights of the working people and the marginlised is often elite arrogance combined with bad public health understanding and is not acceptable. The main question is how to phase it so that it does not lead to a rush of migration and loss of all efforts at physical distancing.
- Re-imposition of lock-downs in a district or cluster of districts should require a rising number of new cases that indicate a R0 value of above an agreed upon threshold (A value of R0 of 1 or less is endemic disease). Disease incidence below that can be managed by ITITT approach alone. Lock-downs in smaller hotspots defined by a physical distance radius must be temporary till a house to house search is carried out, unless the nature of contacts made by the source necessitates it.
- To know the R0 level reliably, the Coronavirus Disease Surveillance Programme (CDSP), which would be part of the IDSP, should be put in place, almost immediately. The current numbers when used as the basis for setting lockdown thresholds are going to be very misleading as increasing numbers due to improved quality of surveillance and case reporting would be interpreted as an increasing epidemic.
- Both state and central governments are trying for lockdown lifting criteria that pertain only to absolute rates of number of cases per million population and without reference to testing protocols and standards of testing and without any proposals as regards to establishing a CDSP. We urge that a lock down should be considered only if there is a sustained increase in cases, and further that the increase occurs only in those who are not already in home or facility quarantine (and therefore indicative of community spread). This allows for withdrawal in an endemic situation, where there are steady new cases, but no accelerated increase in new cases. In other words, where there is still contagion, but there is no epidemic, i.e. we have reached an endemic scenario. This would be the immediate strategic objective. When lock-downs are lifted public transport and workplaces must be opened up in a responsible manner- and phased out carefully. (Reference: JSA-AIPSN statement on proposed extension of national lock-down)
- Across all states and areas irrespective of COVID 19 status measures of physical distancing, ill persons required to wear masks, prevention of large gatherings ( above 20 initially) and basic rules of hygiene would be observed. We may also start testing and certifying for recovered patients who are now testing negative for virus and positive for antibodies and who can therefore be selected for certain tasks.
- The above strategy would hold good till a vaccine is introduced.
Part III: On Access to Medicines and Essential Technologies
- New testing guidelines: New Testing Strategy: The ICMR issued new guidelines on testing allowing rapid antibody based blood test for COVID-19. The ICMR is using this as a strategy for areas reporting clusters (containment zone) and in large migration gatherings and evacuees centres. Resident of areas designated as hot spots and which have reported a large number of cases may have to go through this screening test. According to the guidelines issued by the ICMR, at the facility level, all symptomatic patients with influenza like illness (ILI) are to be tested using antibody tests. The ICMR has given a full treatment protocol regarding testing using antibody test as screening tool and RT-PCR as confirmatory test.
- The Antibody Test Kit: The government is in the process of procuring 5 lakh rapid antibody tests kits from various companies. These tests are useful for their speed as they can suggest whether a person is has COVID19 antibodies within 15 minutes. In most clinical situations where we are testing symptomatic patients with mild, moderate or severe diseases, this test in combination with the clinical setting, would be adequate for a reliable diagnosis that can lead to isolation and defnitive treatment and the use of PPEs by care providers. RT-PCR is desirable but isolation and treatment of the patient and tracing contacts need not wait for that. This also means that testing in clinically symptomatic cases can go down to PHC levels and this offers great advantage in reducing spread of disease ( as these patients would be visiting multiple health centers in search of care) as well as strengthen disease surveillance.
One problem that has been raised with this kit is that it is not useful to detect early cases in the pre-symptomatic phase and will give false negatives in first few days. Also in population studies or in asymptomatic patients a further viral RT-PCR test would be required for confirmation. However after four days of fever (or number of days of fever as recommended after validation studies) in combination with the clinical picture it is very useful and this should be emphasized.
The other problem with this kit is that after the patient recovers the test will remain positive. So it cannot be used for judging recovery. For that we would need a neative viral antigen test. The antibody test is useful to measure past infection and therefore the main tool of population based sero-epidemiological studies..
Governments must report antibody positive patients with clinical symptoms as COVID 19 positive and isolate and treat them as such without waiting for a confirmatory viral antigen test.
- Procurement of Testing Kits: The entry of these rapid antibody testing kits into India would be a game changer. It is not clear as to how many Indian companies would manufacture them and at what scale and what proportion would be imported. These are cheap test at about Rs 400 per test and costs can be lowered further. Scaling up manufacture of this must be priority. The government has provided a list of approved 33 Indian suppliers Rapid / CLIA/ ELISA Corona Kits with condition that they must be first validated by NIV Pune.. All of these 33 are bringing in imported kits, one from Israel, 2 from South Korea, and all the other 30 are importing from China,
As of now though central government and states have placed import orders. The first batch was to have been delivered on April 8th, but as of now.no consignment has arrived, and this is attributed to failing quality tests in China. There are also issues with tendering and payment.
There are also 27 PCR mit suppliers approved. Of these only three are indigenous. Of the rest 10 are from China, 6 are from USA, 4 from South Korea, 2 from Germany and one each from Spain and UK. Given the high needs in their home countries, the actual supply remains to be seen.
There are other cheaper viral antigen tests that have become available ( like the Viral NAT – which is a blood test for viral Nucleic Acid Amplification Tesing ), which is much cheaper than the current PCR test, especially if done on pooled samples. CRISPR-based tools have been developed as a viral tests to test COVID 19 within 30 minutes. These tests are simpler and easier than RT-PCR test with no need for bulky instruments and complicated operationsT This is another option that has to be rapidly explored and scaled up.
The forecasting figures are also not clear. Our annual requirement may be upward of 10 million test kits- and this needs to be factored in when thinking of the manufacturing and import strategy.
- The Scaling up of Testing Services and Payment for Testing: The JSA – AIPSN statement had clearly articulated the need for “free-to-patient” testing. While the government made it free in the public sector it allowed private sector to charge Rs 4500 per test. Fortunately the Supreme Court in an order this week mandated free testing even in the private sector but left modalities undecided. This has forced the government to consider what it should have done in the first place, namely the rate of reimbursement to the private sector for testing. They could negotiate the rate with government based on a quick costing analysis but the private sector would have to provide it free to the patient. Problems of delays in payments can be overcome by giving an advance amount and then refilling it as and when 80% of it is exhausted. The private sector has objected to limiting testing to only those samples/cases which government approves. On this issue, we recommend that the private sector should be allowed to test anyone who by the standard protocol deserves to be tested – and that should definitely include all mild and moderate cases. In the current context, of government limiting its testing to align with its narrative, this autonomy to the private sector to be responsive to the need as certified by its doctors must be respected.
The number of labs doing testing for COVID was only one laboratory even in February. This is now reported to have increased to 223, of which 157 are in the public secor, and and 66 in the private sctor. Daily testing rates were low but now have reached about 10,000 per day.
- PPE: The government statements indicates an order for 1.74 crore PPE sets and N95 and masks. That there is a huge shortage in N95 masks, coveralls and other PPE is too well known to need reiteration here. Doctors, nurses, sanitation staff and other auxiliary staff have been complaining regularly about this, to the extent that gag orders have been issued by government but to no avail since the problem is so severe and threatens the lives of these health care personnel and their families. There is not only a large gap between need and supply, but also a gap between the government’s assertions about plentiful supplies and the reality that most numbers referred to relate to orders rather than actual availability on hand.. There is both lack of transparency and a lack of preparedness. Media reports supported by documentary evidence suggest that most orders for PPE (34 out of 39), were placed only after announcement of the lockdown, a full two months after COVID-19 made its appearance in India, and 24 of these were placed in April 2020. Deliveries both imports and domestic are lagging far behind orders, production against some of which have barely begun. Domestic manufacturers have faced massive pin transportation bottlenecks due to extremely poor implementation of government instructions on the ground, in this case exemptions granted for transport of essential commodities. It is to be noted that public sector undertakings (PSUs) are supplying most of the requirement, despite many years of debasement and under-valuation of their capabilities. Government requires to do its utmost to immediately resolve transportation and supply chain bottlenecks, and extend support to manufacturers to enable rapid scaling up of domestic production.
- Ventilators: The government has announced that it has placed orders for 49,000 ventilators, most of which 10,000 are from BEL, DRDO and the Railways, with some private companeies working with automobile majors. The exact deliverey dates are uncertain..
- Oxygen: A six fold increase in supply of oxygen purposes since February 1st is reported. There is no news on oxygen concentrators.
- Drugs: The government did a flip-flop on Hydroxychloroquine by first banning its export and then hastily withdrawing it in response to US bullying. Consdering the government’s and manufacturers’ statements that there is enough for Indian needs and considering that India commands the market for this medicine, there is indeed a requirement that we help other nations like US and Brazil that have asked for it. We hope we have got a quid pro-quo for import of ventialtors, PPE and testing kits from these nations. On other drugs, it is good news to note that India has joined the WHO solidarity trial.
- Costs of emerging COVID-19 medicines: Many clinical trials are underway looking at efficacy of various drugs for treatment of COVID-19. Prof. Andrew Hill of the University of Liverpool, United Kingdom has published a paper in Journal of Virus Eradication titled “Minimum costs to manufacture new treatments for COVID-19” in which he and his team have calculated costs of these drugs which are repurpose drugs, normally indicated for other diseases. His results show minimum estimated costs of production are US $0.93/day for remdesivir (produced by US multinational corporation Gilead), $1.45/day for favipiravir, $0.08/day for hydroxychloroquine, $0.02/day for chloroquine, $0.10/day for azithromycin, $0.28/day for lopinavir/ritonavir, $0.39/day for sofosbuvir/daclatasvir and $1.09/day for pirfenidone. Costs of production ranged between $0.30 and $31 per treatment course (10–28 days). Going forward, we need to keep a tab on their costs as some of them will be used for COVID-19 depending on the results of clinical trials.
Part IV. Health Systems Strengthening
- On 7th April, the MOHFW issued a Guidance Document on appropriate management of suspect and confirmed cases of COVID-19. The dedicated COVID centres have been divided into 3 types:
- Dedicated COVID Care Centres (CCC) for mild suspected and confirmed cases. These are makeshift facilities. They may be set up in hostels, hotels, schools, stadiums, lodges etc., both public and private. 4000 raliway coaches are also reported as having been readied for the purpose. If need be, existing quarantine facilities could also be converted into CCC. It must also have a dedicated Basic Life Support Ambulance (BLSA) equipped with sufficient oxygen support on 24x7basis
- COVID Health Centres (DCHC) for moderate suspected and confirmed cases. These should either be a full hospital or a separate block in a hospital with preferably separate entry\exit/zoning. These hospitals would have beds with assured Oxygen support. They must have a dedicated BLSA equipped with sufficient oxygen support for ensuring safe transport of a case to a Dedicated COVID Hospital if the symptoms progress from moderate to severe.
- Dedicated COVID Hospitals (DCH) for severe suspected and confirmed cases. It should either be a full hospital or a separate block in a hospital with preferably separate entry\exit. These hospitals would have fully equipped ICUs, Ventilators and beds with assured Oxygen support.
- The official government statement is that it has now readied 520 dedicated COVID hospitals, with nearly 85,000 isolation beds, and 8500 ICU beds. At the next level they also have prepared another 5570 additional health facilities, another 197,400 isolation beds, and a further 36,700 beds. Another 40,000 isolation beds have been prepared from 2500 railway carriages.
- There are reports from every state of such designated centers being created at two levels- for isolation and for ICU care. States are now having to empty out their busiest and most functional of public hospitals and government medical college hospitals to put a DCH in place. The effort to bring a private sector hospital as a COVID19 fell through in many states like Chhattisgarh. But we are not hearing much on the corresponding increases in HR, minor equipment, and major equipment and skills. Moreover, this threatens to have a crippling impact on provision of other routine essential health services, especially in the tertiary level public hospitals.
- While the aggregate numbers of orders placed and hospitals planned are encouraging, reports from many states show a large number of district hospitals which have yet to establish ICU beds and have the necessary equipment and skills for the same. There is concern that in large number of districts, bottlenecks in human resources, skills and supply chains may be inadequate to meet the challenge. Years of lack of preparedness and under-investment cannot be corrected overnight, but at least we can welcome the fact that the government is now seized with the issue.
- Private sector involvement has been varied. Bringing some private hospitals under public authority has been mooted but not done. COVID 19 testing and treatment has been included in PMJAY package but not clear whether this has been availed of. In some states like Tamil Nadu and Mumbai some private hospitals have been accredited for COVID 19 patients but allowed to charge and reports are of very high charges. There are also reports of many accredited hospitals not seeing any COVID 19 likely patients and making no special arrangements to refer them to COVID 19 hospitals. Finally there are also reports of private healthcare having to shut down all services because of healthcare providers becoming infected. There is a clear need to bring select private hospitals completely under a public authority and where they are operating on their own bring them under PMJAY reimbursement protocol for all COVID 19 cases and all cases of SARI. They must be monitored to ensure that their staff is protected and that they do not deny patients the care that is needed.
- One major problem arising is the major decrease in access to essential healthcare services. These include all patients with non-communicable diseases unable to access essential medicines or the care they need from public hospitals and private hospitals, and therefore at much higher risk for complications and mortality. The more critical the patient, like in the case of cancers, renal dialysis, the more severe the problem. On communicable disease front also, postponement and suspension of immunizations services, and difficulties in access services for HIV, TB etc are being reported. Out-Patient and In-Patient services for non-Covid-19 patients in both public and private have been substantially reduced. The decreased access to services is due to four factors:
- a) active elective suspension of services as part of the lock-down- and stay at home instructions.
- b) shifting of staff essential for routine services to COVID 19 related work-especially in states with weaker healthcare systems.
- c) Lack of public transport to reach these services
- d) Many doctors and nurses who are NOT on COVID 19 duty getting COVID 19 infected due to exposure to general patients in whom cases of mild or moderate COVID 19 are intermingled. Once someone tests postive all the staff get isolated or quarantined- shutting whole hospital or much of it down.
The morbidity and mortality due to the decreased access to essential healthcare services is going to increase sharply and exponentially as the lock-down continues. This should be obvious enough, or else such healthcare servies would not have been seen as a fundamental right, but we are yet to come across a modelling exercise that has factored this in.. We had warned against such a situation at the beginning of the lockdown period. However we are seeing the situation getting worse (Ref. Press Statement by JSA Delhi on Denial of Healthcare to Critical Patients and Sudden Discharge from Hospitals without Alternate Arrangements).
Part V: Stigmatisation and Rights violations under the Lockdown
- Stigmatisation and the communalisation of the pandemic: In midst of the ongoing crises, the members of a community who participated in a religious congregation are being hounded and criminalised by the state, police and media. While there may have been lapses by participants of the gathering (such as not reporting that they had visited a Covid-19 affected country), there are instances of people from other communities who have also failed to isolate themselves after coming to India from other countries and have passed on the infection to others. Further, there are reports of religious gatherings of other communities that have taken place as well, some even after the specific congregation11 12 (from rt 2 food). The targeting of Muslims has led to diverting public attention away from the safety precautions that need to be taken to prevent the spread of the virus. Rather than focusing on safety measures, the government, media and the public are instead busy blaming the Muslim community. The attempt to extrapolate the stigma of COVID infection to an entire community, i.e. the Muslim community in India, is nothing short of targeted vilification in a public health crisis. It is also in contravention of the World Health Organization’s advisory issued on 6 April, 2020 that “Countries should not profile novel coronavirus disease (COVID-19) cases in terms of religion or any other criteria”, asking “governments not to politicize the issue and stop profiling people on religious basis.” The advisory also requested people to “never spread names or identity of those affected or under quarantine or their locality on the social media”. This kind of victim-blaming, religious profiling and stigmatising could significantly undermine the public health efforts in Covid-19 epidemic.
- Stigmatisation and suicides and social ostracisation: Even without the above event, this pandemic has become highly stigmatized due to the highly moral tone attached to social distancing and individual responsibility. This pandemic has therefore seen an incredible level of stigmatization, similar to that seen in HIV pandemic or even worse. There are a number of suicides reported because of testing positive or even the fear of testing positive. There are cases of ostracisation by villages and families. There are many reports of people hiding their disease and their symptoms.
- Stigmatisation and attacks on health workers: There are widespread reports from across the country of attacks on health staff and even doctors. Doctors themselves afraid to pass infection to their loved ones are staying away from home, even sleeping in their cars. Resident associations are refusing accommodation to doctors and even turning them out. Doctors and other health workers working with Covid-19 positive patients have been assaulted by people blaming them for spreading coronavirus in their area. The large mobilizational event by government of lighting a lamp and earlier of applauding the health workers for their work by clapping has not made enough difference at the local level where the hate and stigmatization generated by the sub-text has dominated. Health workers are also more at risk due to lack of PPE. When doctors and nurses complain against this, strict gag orders are passed and there are instances of suspension of health workers.
JSA-AIPSN calls for an active end to stigmatization. This requires a major change in media strategy where not only the need but the limitations of social distancing are pointed out. Neither individuals nor communities must be blamed for either getting infected or passing along infection. Social distancing helps but is no guarantee. There have to be active efforts to reach out to aggrieved communities.
- Rising violence on women, girls and children during lockdown: In planning responses to the lockdown, its impact on girls, women and gender-diverse persons has largely been invisible. The burden of domestic and care work which is borne by women has been exacerbated by the lockdown. Given the extreme curtailment of movement, girls and women find no respite affecting their physical and mental health further. Violence – verbal, physical, psychological and economic – against women and girls within homes and institutions are reported to have worsened due to the lockdown, also leading to adverse physical and mental health outcomes. The National Commission for Women reports a sharp rise in number of cases of domestic violence against women and of child abuse. The total number of complaints by women increased from 116 in the first week of March to 257 in the last week of March. In 11 days the Childline India helpline got more than 92,000 SOS calls from children asking for protection from abuse and violence. Reports indicate that police is even more unsympathetic and resistant to registering or acting on complaints. In the Press Release on World Health Day we have urged the government to immediately make arrangements for responding effectively to counter violence related to the pandemic and its control. Calls to the phone helpline should be responded to immediately; a local response team including for providing first aid, counseling should reach the survivor and coordinate all necessary steps and requirements as per the needs of the girl/woman. For example, transport to a safe space or shelter, as may be identified by the girl/woman. All support services, one-stop centres should be functional.
- Rising number of FIRs and police violence for violation of lockdown: Throughout the lockdown we have seen shocking visuals of brutalities against those going out for essential work and there are far too many reports of persecution. Whereas a middle class looking person is likely to have his reason for stepping out accepted, the law is much less flexible with a poor or marginalized person stepping out. Instances of FIRs and police action against such sections including arbitrary actions like seizure of vehicles also abound. We demanded that the government should take strict action against any form of police excess on migrant workers, wage labourers, vendors, the health care workers and others who out of sheer necessity need to break stay-at-home restrictions. (Ref. JSA-AIPSN statement on lock down brutalities)
- Quarantines undermined by poor facilities: There are many reports of conditions of quarantine being so poor that persons break quarantine surreptiously or openly. On hearing about these conditions many deny history of travel or having fever or take paracetomol to hide it- to escape not only stigmatization but also the poor conditions in which they are kept. The risk of infection under such conditions rises for those who are quarantined. We demand that adequate facilities should be provided to those under quarantine, and that the basic rights of the person to food, water, clean sanitation, hygiene, electricity and good health care facilities should not be breached. (Ref. JSA statement on concerns with regard to isolation and quarantine for COVID-19)
- Privacy concerns regarding Apps for surveillance and monitoring during Covid-19
The central Government and many State governments have, or are soon planning to, release mobile Apps as part of their strategy in the fight against the COVID-19 epidemic. This has presumably been done following other countries that have set up similar systems, such as South Korea and Singapore. While in South Korea concerns around privacy and invasive nature of this surveillance were underplayed through a nationalism discourse, Singapore on the other hand was concerned about these issues, and assured users it would not use compile or use data for any purpose other than contact tracing. The various Apps developed and at various stages of being deployed in India seem to be following the South Korean model, believing perhaps that the Indian people and regulatory agencies, maybe even the judiciary, would allow privacy concerns to take a backseat to control of the epidemic.
The Central government’s Aarogya Setu App is supposed to track movements of Covid-19 positive cases and those under quarantine, and even perhaps all people in hotspots and sealed areas. The Aarogya Setu App seeks all personal information such as full name, address and so on, assigns a unique identifier to each user’s phone (with a proposal to also seed it with the Aadhaar number). If a person tests positive, then all her/his contacts are notified and other App users notified if the Covid-19 positive person is nearby. The same company that has provided the Centre and several State governments with facial recognition software for police and other surveillance is providing inputs to integrate such features with Apps being developed in India. While the Aarogya Setu App’s privacy policy states that the data will exist only on anonymised data bases, it would not be difficult to recreate the original data. The policy also states that the data will be retained only for 30 days, but there is the danger that it could easily be available on back-end servers virtually in perpetuity.
State governments are gradually starting to introduce these Apps and also making them compulsory. The Delhi government has decided to use Apps for quarantined persons and for the hotspot containment zones, with the Chief Minister even announcing he is contemplating making use of the Apps compulsory. Punjab has started the COVA App and made it compulsory for companies such as Google and Apple and all social media players to push their customers to download it.
There is little clarity or transparency about which agencies would be running and sharing the App’s data, who can access this data and how long they will be available for. The Indian Apps have little transparency as regards to their privacy policy or potential use of this surveillance data much after the Covid19 epidemic is over and for purposes that have nothing to do with the epidemic. Whatever its supposed immediate benefits during this epidemic, this surveillance trend is deeply worrying for its harmful future potential, especially given India’s steady drift deeper into authoritarian rule.
- Condition of prisons and prisoners: In a joint Statement, JSA and AIPSN expressed deep concern regarding the preparedness of Indian prisons to meet the challenges of the Covid-19 pandemic. (Ref. JSA-AIPSN Recommendations for Prisons in light of the Covid 19 pandemic). JSA and AIPSN made recommendations on decongestion of prisons and for those who cannot be releases, to make COVID related changes and set up a detailed contingency plan.
The Supreme Court is hearing the petition on the issue of overcrowding of prisons and the infrastructure therein. Court has already passed an order that arrangements for transportation of prisoners to their homes or to temporary shelters should be made.
Part VI: Crisis in Livelihoods and the Government Response
Government figures now establish that nearly 50 million short term migrant workers as having lst their work. According to an affidavit filed in the Supreme Court over one million migrant labourers are stuck in shelter homes because they could not make it back home. A mass movement home and then back to their place of work would have to be anticipated once the lock down is lifted. This would need to go along with measures of a minimum wage and a pressure to ensure that these workers are re-employed. Govrnment has announced no plans of how they would reach their homes and how families disrupted and separated by the suddenness of the lock down can re-unite. Clearly the problems of migrant workers continue to be an after-thought. JSA and AIPSN have previously issued a statement on the Economic Package and Demands and deals with the background of the issues mentioned in this section. (Ref. JSA-AIPSN statement on the economic package announced by the government)
- Government relief measures: A number of states have announced relief measures but they are highly inadequate and there are huge gaps in reaching them to those who need it. At the time of writing this, the vast majority may have received little relief except those who got food in shelter homes and community kitchens. Instead of setting up systems for relief measures, the government has left it to the NGOS. In a submission made to the Supreme Court the government stated that in 13 states, NGOs provided food to more people than the state government. A large proportion of migrant and rural workers are expected to further fall below the poverty line due to the Corona virus pandemic that is exacerbated the already existing agricultural and economic crisis. A rapid assessment of the impact of COVID-19 lockdown on migrant workers was undertaken by Jan Sahas who found that the migrants were in near destitute conditions, with high debts and hardly any resources to fall back upon. To overcome the economic slowdown imposed by the lockdown and boost up industrial production, the Government of India is planning to increase the daily working hours from 8 hours to 12 hours as reported by media. Trade unions, like CITU have already expressed their concern that on the issue. Such a move may relieve the employers from paying overtime for extra-working hours, but it would add to the distress of millions of working people in the country, who have been already hit badly due to the lockdown.
The Right To Food Campaign in its latest update it has cited a thread of new reports of atleast 45 deaths, which includes deaths due to hunger, exhaustion and accidents of people walking back to their homes, police atrocities, inability to access medical services and suicides. Along with the migrant workers and rural poor, urban slum dwellers across states are struggling to get access to food and ration as often they do not have ration cards. JSA has endorsed the Right To Food Campaign’s demand to government that PDS should be expanded to cover every individual, irrespective of whether they have a ration card, with 10kg of grain, 1.5kg pulses and 800gms cooking oil per month per person for atleast six months.
- Greater fiscal support to state governments: The central government has notified the release of Rs 11, 092 crores to the states under the State Disaster Risk Management Fund for combating Covid related relief measures. However, the state wise distribution of the funds shows that there is discrepancy in allocation if determined on the basis of number of Covid cases. Maharashtra with highest number of cases has been allocated Rs 1611 crores, while Kerala which had the second largest number of cases till the 3 April received only Rs 157 crore. There have been repeated demands for providing greater fiscal support to the state governments, who are at the frontline of dealing with the situation on the ground.
- Release of foodgrains and pulses: The central government announced in its economic package that ration to priority households under the National Food Security Act would be raised to 10 kg of rice or wheat, and 5 kg of pulses. Issues concerning the policy have been raised in the statement of the Economic Relief Package. According to the latest PIB statement a total of 2 million metric tonnes of food grains have been unloaded by states during the lockdown, while, there was a surplus of total 77 million tonnes with the Food Corporation of India. The pulse reserve with the National Agricultural Cooperative Marketing Federation is also finding it difficult to transport the surplus to the states due to lacking transportation and private milling facilities. The demand for further opening up the food reserves to take care of the food security needs of the growing number of vulnerable people has never been greater. Recently the government has announced that ration would be extended to non-NFSA beneficiaries with ration cards issued by state governments. News coming from the ground shows that this criterion too leaves scope for exclusion. Our demand, as stated above, is for government to provide ration to all who need it.
In conclusion:
We have flagged many of these concerns to the government in our Press Release of April 7th. Now in parallel with the release of this weekly update we shall be releasing three statements which would contain the main demands and recommendations that flow out of this understanding:
-
- Proposed Extension of Nationwide Lockdown: Concerns and Demands by JSA and AIPSN ( http://phmindia.org/2020/04/12/proposed-extension-of-nationwide-lockdown-concerns-and-demands-by-jsa-and-aipsn/ )
- Press Statement on the communalization of the pandemic (in draft stage)
- Press Statement on adverse impact of stigmatization and what must be done to counter this (in draft stage)
Follow for regular updates:
Website www.phmindia.org www.aipsn.net
Twitter @jsa_india
Facebook @janswasthyaabhiyan